Introduction
In today's fast-paced world, accurate and reliable laboratory testing is of utmost importance. Whether it's for medical diagnostics, forensic analysis, or quality control in manufacturing, the validation of laboratory methods ensures that the results obtained are trustworthy and can be used with confidence. In this blog article, we will explore the essential steps involved in laboratory method validation and shed light on the intricacies of this crucial process.
Establishing Method Performance Parameters
Before beginning the validation process, it is essential to define the performance parameters of the laboratory method under consideration. This includes determining the analytical sensitivity, specificity, precision, accuracy, and linearity of the method. By clearly establishing these parameters, the laboratory can set realistic expectations for the method's performance and ensure that it meets the desired criteria.
Designing the Validation Study
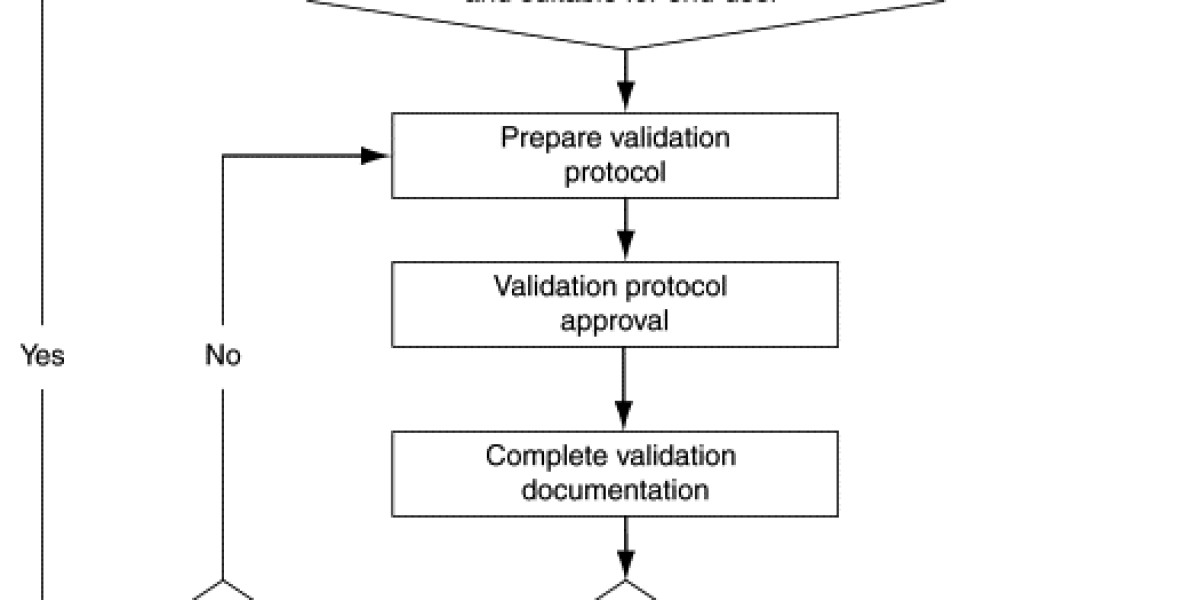
The next step in laboratory method validation is to design a comprehensive validation study. This involves determining the appropriate sample size, selecting representative samples, and establishing a validation protocol that outlines the specific procedures and criteria for evaluating the method's performance. It is crucial to ensure that the study design is robust and capable of providing meaningful results that can be used to make informed decisions.
Conducting the Validation Study
Once the validation study is designed, it is time to conduct the actual validation experiments. This involves performing replicate analyses on the selected samples using the proposed laboratory method. The data obtained from these experiments are then analyzed to assess the method's performance in terms of accuracy, precision, and other relevant parameters. It is essential to meticulously document all the experimental details and observations to maintain transparency and facilitate reproducibility.
Analyzing and Interpreting the Data
After the validation experiments are completed, the data collected needs to be carefully analyzed and interpreted. Statistical methods and data visualization techniques are employed to determine the method's accuracy, precision, and linearity. Any outliers or discrepancies observed during the analysis should be thoroughly investigated to ensure that they do not significantly impact the method's overall performance. The results obtained are then compared against the predefined acceptance criteria to determine whether the method meets the required standards.
Documenting the Validation Results
The final step in laboratory method validation is to document the validation results in a comprehensive validation report. This report should include all the details of the validation study, including the method's performance parameters, the experimental design, the data collected, and the conclusions drawn from the analysis. The report should be written in a clear and concise manner, making it easy for stakeholders to understand the method's strengths and limitations.
Conclusion
Laboratory method validation is a critical process that ensures the accuracy and reliability of laboratory testing. By following the essential steps outlined in this article, laboratories can validate their methods with confidence and provide accurate results to their clients. It is crucial to approach method validation with diligence and attention to detail, as even the smallest errors can have significant consequences. So, remember to establish performance parameters, design a robust study, conduct experiments meticulously, analyze the data rigorously, and document the results comprehensively. With these steps in place, laboratories can uphold the highest standards of quality in their testing processes.